In-Canada distribution of clinical trial materials.
CONTACT USGet in touch to learn more.
Ensuring that your clinical trial materials are handled properly from warehousing, distribution, and collection through destruction is essential to achieving reliable outcomes. At RPS, distribution is one of our most sought-after services not only for the reliability we deliver but also the cost-savings and time-reduction that result from consolidated logistics services when partnering with us.
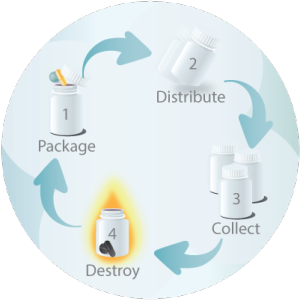 As your clinical material partner, we simplify – and accelerate – your clinical trials. From assistance with time-consuming international documentation through destruction following all regulatory protocols, RPS is your trusted clinical trial partner.
As your clinical material partner, we simplify – and accelerate – your clinical trials. From assistance with time-consuming international documentation through destruction following all regulatory protocols, RPS is your trusted clinical trial partner.
 Ropack Pharma Solutions is positioned to warehouse and distribute even the most sensitive products.
Ropack Pharma Solutions is positioned to warehouse and distribute even the most sensitive products.
Ropack Pharma Solutions is committed to be your trusted manufacturing and packaging partner. Outsource your pharmaceutical or nutraceutical products to us with confidence.